Classification
The contamination of titanium and titanium alloy materials during heating
Submitted by admin on 06/29/2016
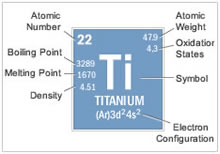
Titanium has high chemical activity, it can react with almost all the elements, such as gas compounds CO, CO2, water vapor, NH4 and many volatile organic compounds at high temperature. In the heating process, the reaction of metal elements and titanium makes the contamination and chemical composition of titanium surface be changed. Some of the gas elements are not only can form the compounds on the surface of titanium, but also it can enter into the metal lattice to form a solid solution.
In heating process of titanium, the results of the interaction with oxygen is to produce the oxide film on the surface. At different heating temperatures, the structure and properties of the oxide film are different. Below 538 degrees Celsius, the oxidation of titanium is in accordance with the law of parabola. However, when it is more than 800 degrees Celsius, the oxide film will be broken down, the oxygen atoms will make the oxide film as the conversion layer into the metal lattice, so that the oxygen content of titanium alloy increases. Oxide film will also be thickened. There is no protective effect of the oxide film at this time. The depth of the pollution layer of titanium alloy (TC4) in the heating furnace of carbon silicon rod is also increased with the increase of heating temperature.
In addition, the oil stain on the workpiece is also the reason to increase the carbon. In the heating process, sweat droplets is also easy to cause the adhesion of chloride, resulting hot salt stress corrosion in the subsequent use. Therefore, it is a very important problem to prevent the contamination of titanium and titanium alloy during the heating process.
Therefore, in order to reduce the impact of various types of atmosphere, the vacuum furnace and the vacuum annealing furnace are generally used, and the inert gas in the vacuum furnace can protect the titanium and titanium alloy materials from pollution.

In heating process of titanium, the results of the interaction with oxygen is to produce the oxide film on the surface. At different heating temperatures, the structure and properties of the oxide film are different. Below 538 degrees Celsius, the oxidation of titanium is in accordance with the law of parabola. However, when it is more than 800 degrees Celsius, the oxide film will be broken down, the oxygen atoms will make the oxide film as the conversion layer into the metal lattice, so that the oxygen content of titanium alloy increases. Oxide film will also be thickened. There is no protective effect of the oxide film at this time. The depth of the pollution layer of titanium alloy (TC4) in the heating furnace of carbon silicon rod is also increased with the increase of heating temperature.
In addition, the oil stain on the workpiece is also the reason to increase the carbon. In the heating process, sweat droplets is also easy to cause the adhesion of chloride, resulting hot salt stress corrosion in the subsequent use. Therefore, it is a very important problem to prevent the contamination of titanium and titanium alloy during the heating process.
Therefore, in order to reduce the impact of various types of atmosphere, the vacuum furnace and the vacuum annealing furnace are generally used, and the inert gas in the vacuum furnace can protect the titanium and titanium alloy materials from pollution.

------分隔线----------------------------




